 3 states of water
3 states of water
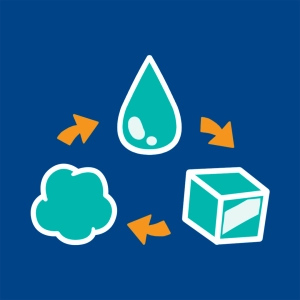
Water can exist in three states (or three phases):
- Solid phase: The particles in a solid are strongly bonded to one another. Ice cubes maintain their form regardless of the container that holds them.
- Liquid phase: The particles are no longer in an ordered state. The bonds between molecules are broken, and the liquid water takes the shape of its container. The particles are very close to one another, and so a liquid is incompressible.
- Gaseous phase: Agitation and disorder are at the maximum level. Water vapor occupies all of the space in a container. The distances between molecules are large. A gas is compressible.
Note that water vapor is invisible.
